We should take the NAD+ precursors
Recognizing the indispensable importance of NAD+, medical scholars have found that its precursors/boosters (NMN or NR) effectively increase the NAD+ levels in the body. To date, within the known scope of knowledge, NAD+ cannot be stably stored at room temperature, nor can it directly increase the body's NAD+ levels through oral ingestion.
We need to take NMN or NR to effectively increase NAD+, not by directly ingesting NAD+. However, there have been many products on the market claiming that NAD+ can be directly ingested. Instead of listening to the marketing gimmicks in the market, let's take a closer look at what the U.S. FDA says.
In 2017, the U.S. FDA Pharmacy Compounding Advisory Committee made the following conclusions about whether Nicotinamide adenine dinucleotide (NAD+) should be included as an effective oral drug ingredient:
NO (Against)
The reason the FDA rejected the inclusion of NAD+ as an effective oral drug ingredient is that NAD+ decomposes at temperatures above -20 degrees Celsius (-20°C). In the presence of alkaline conditions, sunlight, or humidity, NAD+ will also decompose, ultimately breaking down into substances such as nicotinamide riboside, nicotinic acid, and nicotinamide, collectively known as niacin. In other words, NAD+ cannot stably exist in health supplement capsules stored at room temperature. From the moment it leaves -20 degrees Celsius, it is no longer NAD+, but becomes substances like niacin.
The related potential concern is that the human body has an upper limit for the absorption of niacin. The recommended dietary allowance for adults is not to exceed 15mg of niacin per day. (See the diagram below)
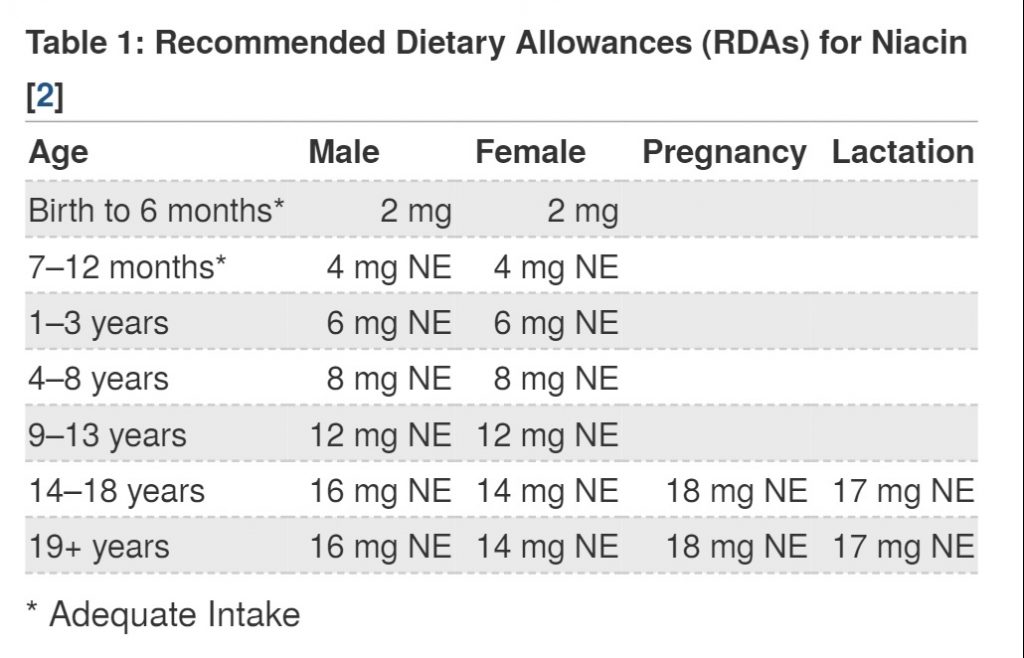
Nicotinamide, one of the components into which NAD+ breaks down, can cause adverse gastrointestinal events if a person absorbs more than 1000mg per day. Intake of amounts greater than 3000mg could potentially lead to liver toxicity.
The FDA suggests that the intake of 5mg of NAD+ is approximately equivalent to the recommended daily allowance for adults of 15mg of niacin.
Clinical evidence shows that taking high doses of Niacin can also potentially cause skin flushing, fever, burning sensations, and itching, a condition known as niacin flush. Professor David Sinclair from Harvard University has publicly mentioned that we do not want to take high doses of nicotinamide as it can inhibit longevity genes such as Sirtuins and PARP, thereby hindering the repair of gene damage. Prof. David Sinclair: "Nicotinamide which is something you don't want to take high doses of because we've showed in my lab many years ago that nicotinamide will inhibit the sirtuins, and PARP as well, and interfere with DNA repair."
If directly ingesting NAD+ were feasible, why do many scholars, Professor Imai Shinichiro, and Professor David Sinclair mention NMN in their research and writings, not NAD+? Please be cautious not to fall into the trap of marketers and jeopardize the health of yourself and your family.
Simultaneously, the UK, Europe, Japan, and other places have not included NAD+ in their official pharmacopoeias. Based on the above considerations, the FDA has rejected the inclusion of NAD+ as an effective drug ingredient under Section 503A of the United States Federal Food, Drug, and Cosmetic Act.
The author recommends that if you are planning to take, or are already taking products advertising NAD+ as a direct supplement, you must understand its ingredients and content clearly. If your body shows any reaction, you must stop taking it immediately and consult your doctor at once.
Reference:
Bulk Drug Substances Used in Compounding Under Section 503A of the FD&C Act
COMPOUNDERS UNDER SECTION 503A OF THE FD&C ACT: QUALITY STANDARDS AND FDA FINDINGS, Pharmacy Compounding Advisory Committee Meeting May 8, 2017
Niacin - Fact Sheet for Health Professionals, National Institutes of Health, U.S. Department of Health & Human Services
